EARLY HTA
In an early Health Technology Assessment (HTA) we will research the minimal economic requirements for the innovation to bring added value, compared to the current standard of care. We will focus on the intended costs and the intended effects of an innovation. An early HTA provides an early estimation of the change in healthcare costs after implementing the innovation.

PAPER PROTOTYPING
In deze ontwikkelstap worden op papier uitgewerkte ontwerpscenario's voorgelegd aan (Alpha Usability) of ontwikkelt met (paper prototyping) kleine groepen eindgebruikers. In deze sessies worden patienten uitgedaagd te interacteren met de papiere protypes, bijvoorbeeld door het uitvoeren van taken. Dit is de volgende stap in het creeeren van kaders voor het ontwikkelproces en is essentieel in het voorkomen van onnodige R&D kosten.

IDEATION / DESIGN THINKING
After careful consideration of the mechanism of action and the end-user’s expectations, several potential solutions will be identified. This is a creative and hands-on exercise.

PROBLEM/NEEDS ANALYSE
Needs, perceptions and capabilities of the intended end user need to be clear. We will consult experts and intended users to demonstrate how the innovation will bring effect to a certain target group. This analysis is an important step as it reveals the user requirements, which are crucial for successful development.

LITERATURE REVIEW
Using a systematic literature review, we will identify the current scientific evidence that can substantiate the mechanism of action of the innovation. This will provide the innovator an idea of the theoretical chance of the innovation to be effective and as such to further build the business case. Additionally, it provides valuable input for what studies are needed to fill the identified gaps in the knowledge base to further substantiate the mechanism of action.
APPLICATION IDENTIFICATION
What would be the best context to apply the innovation? In which patient group or care pathway will the innovation generate the most impact? During this application identification we will introduce the innovator to experts in potential application areas to understand how they would welcome this type of innovation in their care processes and to eventually identify the most favorable context of application.

CONTEXT & CURRENT PRACTICE ANALYSE
During this analysis, an independent portfolio will be built that clarifies which (unmet) needs will be addressed with the innovation. Using literature research, interviews and focus groups it will be assessed which problems can be addressed by the innovation, and how this compares to the current standard.

DEMONSTRATION OF THE MECHANISM OF ACTION
In this analysis a causal path will be drawn to clarify how the innovation will result in the intended positive effects. These pathways are crucial to determine consecutive studies and relevant outcomes to study to eventually demonstrate the benefits of the innovation.

EARLY HTA
In an early Health Technology Assessment (HTA) we will research the minimal economic requirements for the innovation to bring added value, compared to the current standard of care. We will focus on the intended costs and the intended effects of an innovation. An early HTA provides an early estimation of the change in healthcare costs after implementing the innovation.

FEASIBILITY TESTING
A feasibility study assesses the acceptance of an innovation for a longer period of usage, in the intended context. Using a mixed method of qualitative and quantitative methods, several aspects will be measured, such as: acceptance, interest, implementation, practicability, adaptation, integration, expansion and preliminary effectiveness.

USABILITY TESTING
In a usability test we evaluate the usability and user friendliness of an innovation with end users. Using for example (video) observation and qualitative research, we will be able to quantify how users actually interact with and experience the innovation. This study will give recommendations for optimization and further implementation.

HTA
In this extensive quantitative analysis, we will use prospective study data, literature data and expert opinion. Optionally we develop a model to answer several cost effectiveness questions or scenarios. For example: what are the additional costs per gained life year? Does the prevention of a complication result in cost savings?

CLINICAL STUDY
In a clinical study, the (clinical) performance, efficacy, effectiveness and/or efficiency of the innovation will be studied. In designing and performing the clinical study, several elements need to be addressed such as the need for a control group, randomization, follow-up time. We will consider the regulatory and ethical requirements as well as the logistics.

METHODOLOGY CLINICAL STUDY
We offer consultancy and advisory activities in setting up a clinical study and writing a study protocol. We can help to answer questions like: What would be the most efficient and appropriate design for the study to validly answer my research question? What aspects need to be covered by the study protocol to comply with rules and regulations? How many participants do I need?

POST-MARKET SURVEILLANCE
Post market surveillance is a set of activities to monitor the safety and performance of an innovation, once implemented. THINC. has the research and data management techniques (European Guidelines: 93/42EEG) to determine the usability, safety and vigilance of an innovation after implementation. These analyses come with extensive recommendations on product and process improvement.
You are a starting healthcare innovator who wants to make an impact in healthcare:
- You still need CE certification.
- You want to know the potential health and economic effectiveness of your innovation
A voucher of up to 5,000 euros is available for a THINC. First product that helps you take a first step in this direction.
THINC. First – Voucher
Jij bent een startende zorginnovator, die impact wil maken in de zorg. Je hebt nog een CE certificering nodig. Of je wilt weten wat de potentiële gezondheids- en economische effectiviteit van je innovatie is.
THINC. kan je hierbij ondersteunen met validatie van je innovatie. Om je financieel tegemoet te komen, bieden we voor een eerste stap in deze richting een THINC First product aan die voor de helft gesubsidieerd wordt door een voucher van Health Holland.
![]()
Wat zijn de voorwaarden:
- Je draagt met je innovatie bij aan een van de 4 missies, opgesteld door het ministerie van Volksgezondheid, Welzijn en Sport. CONTINUE voor meer informatie over deze missies
- Maximum project omvang is 10.000 euro (excl. BTW)
- Je draagt 50% van het project financieel zelf bij
Wat bieden we:
- THINC. First; Health Technology Assessment
- THINC. First; Clinical Evaluation
Health Holland, ROM Utrecht en THINC. werken samen om deze voucher mogelijk te maken.
Wil je gebruik maken van deze voucher of meer weten?
Neem dan contact op met Gerdi Janssen (Sr. Business Developer THINC.)
via volgende mailadres: g.j.janssen-4@umcutrecht.nl
Minddistrict

Minddistrict
THINC. heeft Minddistrict ondersteunt met het realiseren van CE-markering klinische documentatie volgens de MDR. Onze activiteiten hebben geresulteerd in een verdiept inzicht in de claims van Minddistrict, de kwaliteit van de Clinical Evaluation Plan (CEPs) en mogelijkheden om bestaande kennislacunes op een geldige manier aan te vullen. Dit heeft geleid tot een duidelijke richting voor zowel huidige als toekomstige CEPs. Ons gezamenlijke doel is om te komen tot een ‘portfolio of evidence’ waarmee de claims voor CE-markering op overtuigende wijze zijn onderbouwd, waarmee relevante stakeholders worden overtuigd.
Minddistrict is een softwareplatform voor zorgprofessionals. Het stelt hen in staat om mensen op een gepersonaliseerde manier te ondersteunen in gedragsverandering door middel van eHealth-technologie. Modules, dagboeken en andere onderdelen in Minddistrict’s platform zijn transdiagnostisch: ze zijn niet per se gericht op het stellen van een diagnose, maar kunnen ook klachtgericht, therapeutisch, ingezet kunnen worden.
StrokeViewer

StrokeViewer
THINC. created a business case for Nico Lab for hospitals and supported them in discussions with health insurers about reimbursement. A logic model was used to map the care pathway. This model was presented to experts and based on their feedback we created a health economic model using data from literature and the first pilot studies of Nicolab. Based on the model, an app was created that allows Nicolab to adjust various input parameters to local hospital context, allowing Nicolab to show specific potential customers what the expected impact is for the particular hospital.
Nico lab has developed the StrokeViewer product. It analyzes CT scans of incoming patients where the patient is suspected of having a stroke. When a stroke is identified, the images are immediately forwarded to the appropriate physicians with a notification. The goal of the innovation is to get more patients to the right center at once and receive the best appropriate care as quickly as possible.

EARLY HTA
In an early Health Technology Assessment (HTA) we will research the minimal economic requirements for the innovation to bring added value, compared to the current standard of care. We will focus on the intended costs and the intended effects of an innovation. An early HTA provides an early estimation of the change in healthcare costs after implementing the innovation.
Regenexx

Regenexx
Regenexx biedt geavanceerde stamcelbehandelingen die beschikbaar zijn in de Verenigde Staten, een alternatief voor orthopedische operaties. Door de jarenlange ervaring heeft Regenexx inmiddels een uitgebreide database met resultaten. Na een succesvol eerste project voor knieartrose hebben we in opdracht van Regenexx eenzelfde analyse ook uitgevoerd voor problemen met het schoudergewricht en voor voorste kruisband beschadigingen.
Op dit moment zijn er vragen met betrekking tot kostenbesparingen, economische evaluaties van stamcelbehandeling en vergoeding. Een overzicht werd gemaakt van de behoeften van besluitvormers en deze werden geprojecteerd op de huidige beschikbare gegevens om hiaten in het bewijs te identificeren. Een kosteneffectiviteitsanalyse is uitgevoerd, waarbij gebruik is gemaakt van een standaard HTA-methoden. Tenslotte werd informatie verzameld over hoe de resultaten op maat gemaakt zouden kunnen worden om zo goed mogelijk aan te sluiten bij de behoefte van zelfverzekerde bedrijven.

EARLY HTA
In an early Health Technology Assessment (HTA) we will research the minimal economic requirements for the innovation to bring added value, compared to the current standard of care. We will focus on the intended costs and the intended effects of an innovation. An early HTA provides an early estimation of the change in healthcare costs after implementing the innovation.
Noscendo GmbH

Noscendo GmbH
Noscendo DISQVER provides a new way to identify pathogens based on algorithms and next generation sequencing. This enables faster and more accurate diagnosis of acute and severe infections.
THINC. heeft in opdracht van Noscendo aan de ontwikkeling van een vroeg gezondheidseconomisch model gewerkt. Met dit model hebben we de verwachtte toegevoegde waarde van op DISQVER bij de diagnostiek van sepsis aangetoond. Noscendo kan de analyse gebruiken voor verschillende specifieke populaties in verschillende landen en kunnen zij de verwachte toegevoegde waarde van DISQVER aan verschillende potentiële klanten en investeerders laten zien.

EARLY HTA
In an early Health Technology Assessment (HTA) we will research the minimal economic requirements for the innovation to bring added value, compared to the current standard of care. We will focus on the intended costs and the intended effects of an innovation. An early HTA provides an early estimation of the change in healthcare costs after implementing the innovation.
Clear.

Clear.
It is believed that the glycemic response after a meal (postprandial) plays an important role in health and disease. Precision nutrition methods have the ability to personalize an individual's diet to minimize postprandial glycemic responses measured by continuous glucose monitoring.
THINC. was asked to model the causal pathway and specify Clear's claims for different intended use scenarios. 3 different clinical evaluation scenarios were performed to eliminate different types of uncertainties for this mechanism of action.
Clear B.V. has developed The Clear Health Program that identifies an individual's glycemic response to different types of food. Based on the findings, users receive a personalized nutrition plan and guidance through the app.

DEMONSTRATION OF THE MECHANISM OF ACTION
In this analysis a causal path will be drawn to clarify how the innovation will result in the intended positive effects. These pathways are crucial to determine consecutive studies and relevant outcomes to study to eventually demonstrate the benefits of the innovation.

METHODOLOGY CLINICAL STUDY
We offer consultancy and advisory activities in setting up a clinical study and writing a study protocol. We can help to answer questions like: What would be the most efficient and appropriate design for the study to validly answer my research question? What aspects need to be covered by the study protocol to comply with rules and regulations? How many participants do I need?
OLVG Bariatrie

OLVG Bariatrie
Research shows that stomach reduction surgery in day care is safe and provides shorter wait times. THINC. has been asked to investigate how the new care pathway is perceived by patients themselves and/or whether they are satisfied with the care. With the knowledge of and experience with qualitative research methods that explore the patient perspective, care can be further improved and tailored to what is important to the patient
OLVG Bariatrics is a new care pathway for patients undergoing stomach reduction surgery. They do not have to stay in the hospital after their surgery, but may go home the same day under certain conditions. This is called a stomach reduction surgery in day treatment.

USABILITY TESTING
In a usability test we evaluate the usability and user friendliness of an innovation with end users. Using for example (video) observation and qualitative research, we will be able to quantify how users actually interact with and experience the innovation. This study will give recommendations for optimization and further implementation.

FEASIBILITY TESTING
A feasibility study assesses the acceptance of an innovation for a longer period of usage, in the intended context. Using a mixed method of qualitative and quantitative methods, several aspects will be measured, such as: acceptance, interest, implementation, practicability, adaptation, integration, expansion and preliminary effectiveness.
Protocol Feasibility

Protocol Feasibility
THINC. has been asked to set up a pilot feasibility study to gain insight into the experiences surrounding the use of continuous monitoring solutions in practice. This protocol will be used to gain insight into the impact and what the success and improvement points are for implementation.
Fastfocus is a continuous monitoring solution that enables more efficient, sustainable and successful healthcare operations.

FEASIBILITY TESTING
A feasibility study assesses the acceptance of an innovation for a longer period of usage, in the intended context. Using a mixed method of qualitative and quantitative methods, several aspects will be measured, such as: acceptance, interest, implementation, practicability, adaptation, integration, expansion and preliminary effectiveness.
Medicinfo

Medicinfo
Medicinfo has a desire to expand its hybrid care offering, to have the digital anamnesis around entry complaints externally reviewed by experts, and to look at its dataset with critical and constructive eyes to prepare it for next steps, including in the field of AI (artificial intelligence).
THINC. offers Medicinfo the opportunity to verify medical content around the entry complaints with independent experts, with THINC. investigator simultaneously reviewing treatment areas. Content in the digital system is reviewed by triagists and physicians for urgency assignment and completeness, relevance and wording of the history.

USABILITY TESTING
In a usability test we evaluate the usability and user friendliness of an innovation with end users. Using for example (video) observation and qualitative research, we will be able to quantify how users actually interact with and experience the innovation. This study will give recommendations for optimization and further implementation.

FEASIBILITY TESTING
A feasibility study assesses the acceptance of an innovation for a longer period of usage, in the intended context. Using a mixed method of qualitative and quantitative methods, several aspects will be measured, such as: acceptance, interest, implementation, practicability, adaptation, integration, expansion and preliminary effectiveness.
Bilihome

Bilihome
THINC. supported Bilihome in usability evaluation within CE certification (MDR).
Bilihome aims to support newborns with jaundice and their parents. The portable solution enables optimal blue light therapy and helps babies recover in the right environment.

USABILITY TESTING
In a usability test we evaluate the usability and user friendliness of an innovation with end users. Using for example (video) observation and qualitative research, we will be able to quantify how users actually interact with and experience the innovation. This study will give recommendations for optimization and further implementation.
Wachtenwaard

Wachtenwaard
Last year, Lente W.'s innovation "Waiting Worth" was the winner of Dutch Hacking Health. The prize was a THINC. First session with THINC.
Meanwhile, a pilot has started within Altrecht to investigate how 'WachtenWaard' can reduce the waiting list problem in the GGZ. They are now ready to cash in on the THINC. First session to cash in.
During the THINC. First session we discussed the current status of the pilot and what (research) steps can be taken to achieve structural funding and embedding of 'WachtenWaard' in daily practice.
Baby@home

Baby@home
THINC has been asked by the neonatology department to design and conduct a scientific study regarding evaluation of parents' experiences.
Baby@Home is an app developed by the WKZ neonatology department in collaboration with Luscii Healthtech. The app allows premature babies to go home earlier. Parents enter measurement data at home and these are viewed daily by nurses and neonatologists at the WKZ, to closely monitor the baby's development. Also, parents can have low-threshold consultations and ask their questions.

FEASIBILITY TESTING
A feasibility study assesses the acceptance of an innovation for a longer period of usage, in the intended context. Using a mixed method of qualitative and quantitative methods, several aspects will be measured, such as: acceptance, interest, implementation, practicability, adaptation, integration, expansion and preliminary effectiveness.
Vitaly platform

Vitaly platform
Within THINC, we are placing a magnifying glass over the rollout of the Vitaly platform. We are evaluating the acceptance, implementation, feasibility and preliminary efficacy of Vitaly in supporting these MDOs. The key process parameters being evaluated are preparation and discussion time, delay rate, number of cases discussed and preparation errors. In addition, clinician satisfaction will be evaluated.
Within the #DatasharingMiddenNederland project, the choice was made to digitally support the MDOs with the Vitaly platform of Parsek Group, part of Open Line. This is expected to boost the quality and efficiency of these MDOs.

USABILITY TESTING
In a usability test we evaluate the usability and user friendliness of an innovation with end users. Using for example (video) observation and qualitative research, we will be able to quantify how users actually interact with and experience the innovation. This study will give recommendations for optimization and further implementation.

FEASIBILITY TESTING
A feasibility study assesses the acceptance of an innovation for a longer period of usage, in the intended context. Using a mixed method of qualitative and quantitative methods, several aspects will be measured, such as: acceptance, interest, implementation, practicability, adaptation, integration, expansion and preliminary effectiveness.
Virtual Fracture Care

Virtual Fracture Care
Researchers from St. Antonius Hospital, OLVG and THINC. came together to triangulate the results of a study of healthcare professionals' experiences with Virtual Fracture Care (VFC). THINC. took care of the qualitative part of the data collection and analysis. In the triangulation session, quantitative results from questionnaires and qualitative results from interviews were juxtaposed and compared. For example, the questionnaires asked for a rating for satisfaction with VFC. The interviews specifically asked about the reason for a particular grade and how it could be increased. This provided valuable insights that help Antonius Hospital understand the surveyed experiences even better. This leads to more insight into the impact it has in practice and what the success and improvement points are in this.
Nb. Virtual Fracture Care method: this means that patients with relatively simple and stable injuries no longer receive an outpatient follow-up checkup after visiting the hospital, but receive all information in the form of an app to recover at home.

FEASIBILITY TESTING
A feasibility study assesses the acceptance of an innovation for a longer period of usage, in the intended context. Using a mixed method of qualitative and quantitative methods, several aspects will be measured, such as: acceptance, interest, implementation, practicability, adaptation, integration, expansion and preliminary effectiveness.
Scinvivo

Scinvivo
THINC ontwikkelt voor Scinvivo, een economisch model om de omvang van potentiële klinische en uiteindelijke economische effect voor de katheter in detail te kwantificeren in vergelijking met de huidige diagnostistische methode. Dit is nodig om investeerders te kunnen informeren en informatie op te kunnen halen waarmee zij hun vervolgstudies nog beter kunnen ontwerpen.
Scinvivo believes that the current diagnosis of bladder cancer can be improved with new imaging technologies. Therefore, Scinvivo is developing an imaging catheter that allows the urologist to visualize the structure of the bladder wall. Current diagnostic methods are suboptimal because the urologist must base his decision on incomplete visual information.

EARLY HTA
In an early Health Technology Assessment (HTA) we will research the minimal economic requirements for the innovation to bring added value, compared to the current standard of care. We will focus on the intended costs and the intended effects of an innovation. An early HTA provides an early estimation of the change in healthcare costs after implementing the innovation.
JINSHAN

JINSHAN
THINC maps Dutch diagnostic pathways, current practice and variations for gastric pathology detection and diagnostic follow-up. Delivers a stepwise approach to scientific services in support of JINSHAN's needs. Thanks in part to interviews with Key Opinion Leaders in the field of gastric diagnostics, we gained insight into where the opportunities lie for innovation in the Dutch healthcare landscape (room for improvement in Dutch Helathcare).
JINSHAN has developed a fully automatic magnetic controlled capsule endoscopy (MCE) that does not require a human operator to control the movement of the capsule.
Spatium

Spatium
THINC. Healthcare guides Spatium Medical in making an evidence-based choice (room for economic improvement). We do this using scientific literature and input from experts such as surgeons and anesthesiologists. THINC investigates for which patient population the insufflator gives the most added value and where the most costs can be saved. How does it fit into the current healthcare landscape?
Spatium Medical has developed an insufflator that, among other things, can apply personalized and stable pressure, which can provide an improvement in patient health. Examples where potential added value could begin to occur include shorter surgery time, shorter intubation time and improvement in pain after surgery.
YON E I

YON E I
THINC has advised YON.E to what extent their innovation concerns a medical device and what conditions regarding validations are involved (CE certification support). Also, we gave recommendations on the type of validation studies that we can perform together, which are needed during an MDR process. All this to create the most convincing portfolio of evidence possible for YON.E. Based on our input, they were able to bring investors along with the necessary investment involved. We are very pleased to be able to provide crucial advice at this early stage and to be able to work with them as a long-term validation partner.
YON.E aims to create vaginal comfort worldwide, one device, one person at a time, by giving women back control of their physical and emotional well-being.
Cordys
Cordys
THINC. is currently working with researchers at the Department of Cardiology at UMC Utrecht to explore the health economic effects of their ECG Triage Algorithm (room for economic improvement).
Wat als het mogelijk is om een deel van de ECG’s automatisch te laten evalueren? Wat zou het aan tijd en geld schelen? En wat zou dat voor de patient opleveren? THINC werkt aan het beantwoorden van deze vragen. Een Early HTA wordt uitgevoerd om in kaart te brengen waar de kansen liggen voor hun product en hen te informeren over de vervolgstappen op klinische evaluatie en business development.
In een eerste slag hebben we experts in het veld gesproken en de eerste resultaten laten een veelbelovende besparing in tijd en dus geld zien. De volgende stap is het in kaart brengen van de mogelijke gezondheidswinst voor de patiënt.

HTA
In this extensive quantitative analysis, we will use prospective study data, literature data and expert opinion. Optionally we develop a model to answer several cost effectiveness questions or scenarios. For example: what are the additional costs per gained life year? Does the prevention of a complication result in cost savings?
PhoenixDX

PhoenixDX
THINC. assists PhoenixDX in identifying the most valuable clinical applications for their innovation. Specific components within this assignment are: (1) logic models summarizing the different steps through which the innovation leads to patient or healthcare benefits; (2) overview of the most important clinical applications with expert opinions on the desirability and conditions to be successful; (3) exploration of the market size of the most relevant clinical applications
PhoenixDX, een biotech-bedrijf uit Engeland, heeft de GENIXDX innovation ontwikkeld. GENIXDX is een point-of-care test die snel en betrouwbaar verwekkers van een infectie kan aantonen. Er kan specifiek voor één verwekker getest worden of voor een panel van verwekkers. De huidige versie is gericht op het aantonen van SARS-CoV-2, Influenza A, en Influenza B bij personen verdacht voor een COVID-19 infectie. De technologie kan uitgebreid worden naar de detectie van andere virussen, maar ook bacteriën.

POST-MARKET SURVEILLANCE
Post market surveillance is a set of activities to monitor the safety and performance of an innovation, once implemented. THINC. has the research and data management techniques (European Guidelines: 93/42EEG) to determine the usability, safety and vigilance of an innovation after implementation. These analyses come with extensive recommendations on product and process improvement.

METHODOLOGY CLINICAL STUDY
We offer consultancy and advisory activities in setting up a clinical study and writing a study protocol. We can help to answer questions like: What would be the most efficient and appropriate design for the study to validly answer my research question? What aspects need to be covered by the study protocol to comply with rules and regulations? How many participants do I need?
Leontienhuis

Leontienhuis
THINC. conducted an innovation assessment to identify the operating mechanism by which the Leontien House is trying to achieve its goals, to test the plausibility of the Leontien House achieving its goals through its current activities, and to identify opportunities for additional research to demonstrate with greater certainty whether the Leontien House is promoting health benefits and cost savings.
The Leontien House aims to be an accessible and safe drop-in center for people with an eating disorder. In addition to stimulating optimal coping with the eating disorder in a low-threshold and non-committal manner, it aims to motivate people who normally stay out of treatment to take the first step towards treatment, to stay in treatment and not to fall back into old behavior after treatment.

PROBLEM/NEEDS ANALYSE
Needs, perceptions and capabilities of the intended end user need to be clear. We will consult experts and intended users to demonstrate how the innovation will bring effect to a certain target group. This analysis is an important step as it reveals the user requirements, which are crucial for successful development.

POST-MARKET SURVEILLANCE
Post market surveillance is a set of activities to monitor the safety and performance of an innovation, once implemented. THINC. has the research and data management techniques (European Guidelines: 93/42EEG) to determine the usability, safety and vigilance of an innovation after implementation. These analyses come with extensive recommendations on product and process improvement.
Ares Analytics
Ares Analytics
THINC. in collaboration with Ares Analytics and clinical experts, has developed an early evaluation study that will further assist in selecting the most valuable components for further research and development.
Ares Analytics aims to develop a low-cost, lightweight and non-invasive wearable that continuously tracks training intensity using data science. Athletes are constantly looking for ways to assess and further improve their performance. Information about training intensity and fatigue are crucial for an athlete to train efficiently and reduce the risk of sports-related injuries. The level of fatigue also determines the length of the optimal recovery period before continuing training. This is true not only for athletes, but also for specific types of patients.

CLINICAL STUDY
In a clinical study, the (clinical) performance, efficacy, effectiveness and/or efficiency of the innovation will be studied. In designing and performing the clinical study, several elements need to be addressed such as the need for a control group, randomization, follow-up time. We will consider the regulatory and ethical requirements as well as the logistics.
STRIDE

STRIDE
THINC. heeft een THINC first assessment gedaan om inzicht te krijgen in het Product & Inzet’ (o.a. claims en populatie), ‘CE-certificering’ (o.a. intended use en risicoclassificatie), ‘Stakeholders’ (o.a. welke stakeholders zijn het meest relevant en wat zijn hun interesses), ‘Evidence & Studies’ (o.a. welk wetenschappelijk bewijs is er en wat moet nog worden geproduceerd), en ‘Implementatie’ (o.a. barrières en facilitators tot succesvolle implementatie). Na een ronde tafel gesprek met relevante stakeholders (o.a. patienten, zorgverzekeraars, clinici, CE-exprt, Zorginstituut Nederland) zijn concrete aanbevelingen voor de toekomst van STRIDE geformuleerd.
The STRIDE innovation consists of two components: a prognostic algorithm and a functional in vitro test. The STRIDE innovation is a decision-support tool to improve matching between an available donor kidney and a potential recipient.

METHODOLOGY CLINICAL STUDY
We offer consultancy and advisory activities in setting up a clinical study and writing a study protocol. We can help to answer questions like: What would be the most efficient and appropriate design for the study to validly answer my research question? What aspects need to be covered by the study protocol to comply with rules and regulations? How many participants do I need?
Philips Image Guided Therapies
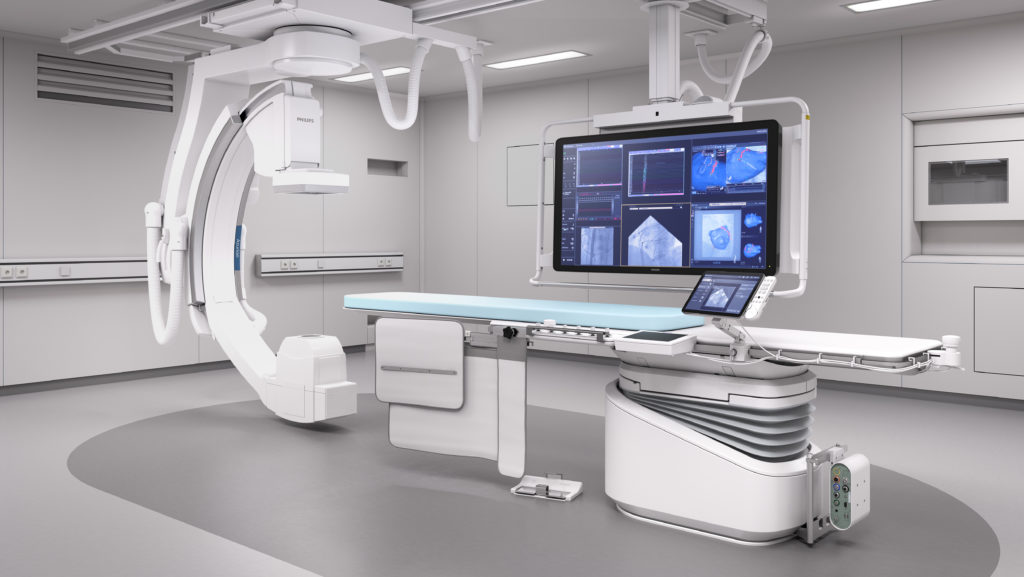
Philips Image Guided Therapies
THINC. conducted a systematic review to identify the most valid and applicable prediction models that relate contrast volume and other clinical variables to acute kidney injury and other relevant outcomes. In addition, THINC created a health economic model identifying the best models based on using the literature to determine the costs and effects of current care and care after implementation of DCR.
Philips Image Guided Therapies developed a new software package for their angiography and percutaneous coronary intervention products, the Philips Dynamic Coronary Roadmap (DCR). With this software package, an image of the coronary arteries is created with X-rays and a contrast agent projected over the X-rays taken during the subsequent procedure. It is expected that cardiologists and/or radiologists will be able to perform complex coronary interventions with less contrast agent. The use of contrast agents in interventional cardiology is associated with acute renal injury and, in some cases, long-term renal failure requiring dialysis. Therefore, the question was whether a reduction in contrast volume is likely to lead to a decrease in acute kidney injury and dialysis incidence. In addition, Philips was interested in assessing whether a possible decrease in actuu renal failure and dialysis incidence would also result in cost savings for hospitals practicing interventional cardiology.

EARLY HTA
In an early Health Technology Assessment (HTA) we will research the minimal economic requirements for the innovation to bring added value, compared to the current standard of care. We will focus on the intended costs and the intended effects of an innovation. An early HTA provides an early estimation of the change in healthcare costs after implementing the innovation.
Quarantaine coach

Quarantaine coach
THINC evaluated the pilot with quarantine coaches. How great was the need for the quarantine coaches and how did the people coached experience the help provided.
In February 2021, the GGDs, the Red Cross and the Ministry of Health, Welfare and Sport launched a pilot with "quarantine coaches" in the Drenthe and Kennemerland regions. Quarantine coaches support people who experience psychological or practical difficulties during quarantine.

DEMONSTRATION OF THE MECHANISM OF ACTION
In this analysis a causal path will be drawn to clarify how the innovation will result in the intended positive effects. These pathways are crucial to determine consecutive studies and relevant outcomes to study to eventually demonstrate the benefits of the innovation.

USABILITY TESTING
In a usability test we evaluate the usability and user friendliness of an innovation with end users. Using for example (video) observation and qualitative research, we will be able to quantify how users actually interact with and experience the innovation. This study will give recommendations for optimization and further implementation.
spreekuur.nl
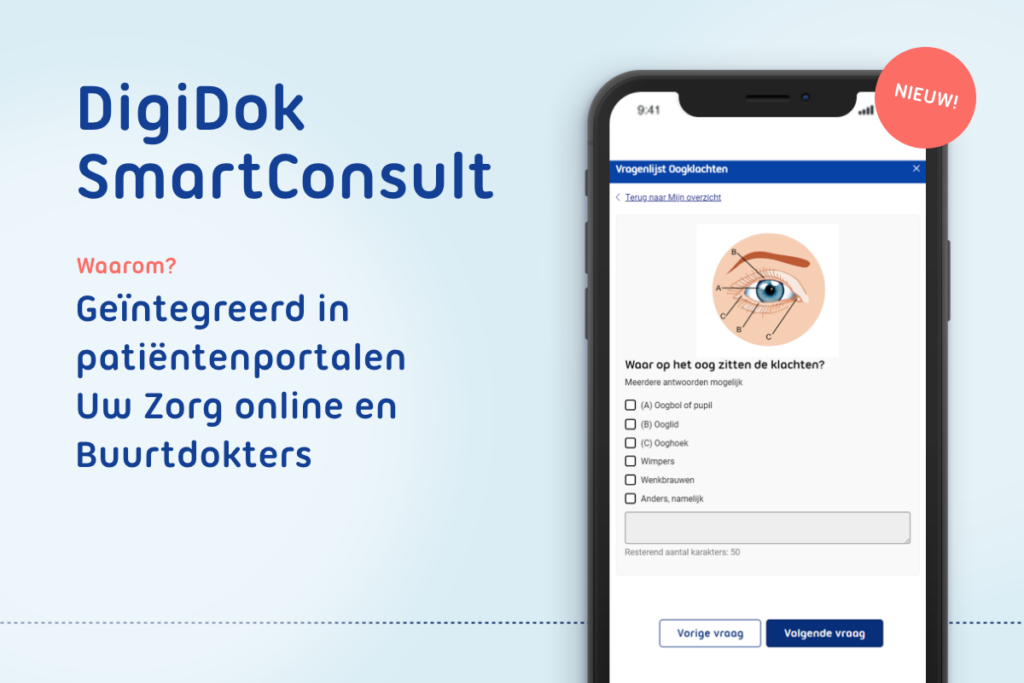
spreekuur.nl
THINC validated the questionnaires used during several individual sessions with trainees and general practitioners. This included assessing the triage and anamnesis questions for completeness, relevance and unambiguity.
Spreekuur.nl is an initiative of DigiDok and Topicus. It is the first digital general practitioner clinic in the Netherlands. For various treatment areas (e.g., sore throat, back and neck pain and insect bites), patients can prepare their consultation themselves by filling in triage and anamnesis questions and adding a photo and/or video. General practitioners are presented with this information conveniently via a digital system and can complete the consultation smoothly via chat or video call.

USABILITY TESTING
In a usability test we evaluate the usability and user friendliness of an innovation with end users. Using for example (video) observation and qualitative research, we will be able to quantify how users actually interact with and experience the innovation. This study will give recommendations for optimization and further implementation.

DEMONSTRATION OF THE MECHANISM OF ACTION
In this analysis a causal path will be drawn to clarify how the innovation will result in the intended positive effects. These pathways are crucial to determine consecutive studies and relevant outcomes to study to eventually demonstrate the benefits of the innovation.
PCaVision

PCaVision
Due in part to a THINC. first assessment, Angiogenesis Analytics secures a major European grant to diagnose prostate cancer with their innovation, PCaVision!
THINC. explored the potential value of PCaVision to generate clinical benefits and/or to be cost-effective. We explored possible intended roles for PCaVision, claims about potential benefits, and how to build a compelling portfolio of evidence to support these claims. The output of this helped win a large Horizon Europe grant (EIC).
The company Angiogenesis Analytics has developed an integrated system for performing and analyzing 3D multiparametric ultrasound images called PCaVision. PCaVision enables the measurement and visualization of angiogenesis in the prostate, a key feature of cancerl development.

EARLY HTA
In an early Health Technology Assessment (HTA) we will research the minimal economic requirements for the innovation to bring added value, compared to the current standard of care. We will focus on the intended costs and the intended effects of an innovation. An early HTA provides an early estimation of the change in healthcare costs after implementing the innovation.
Haermonics Flush
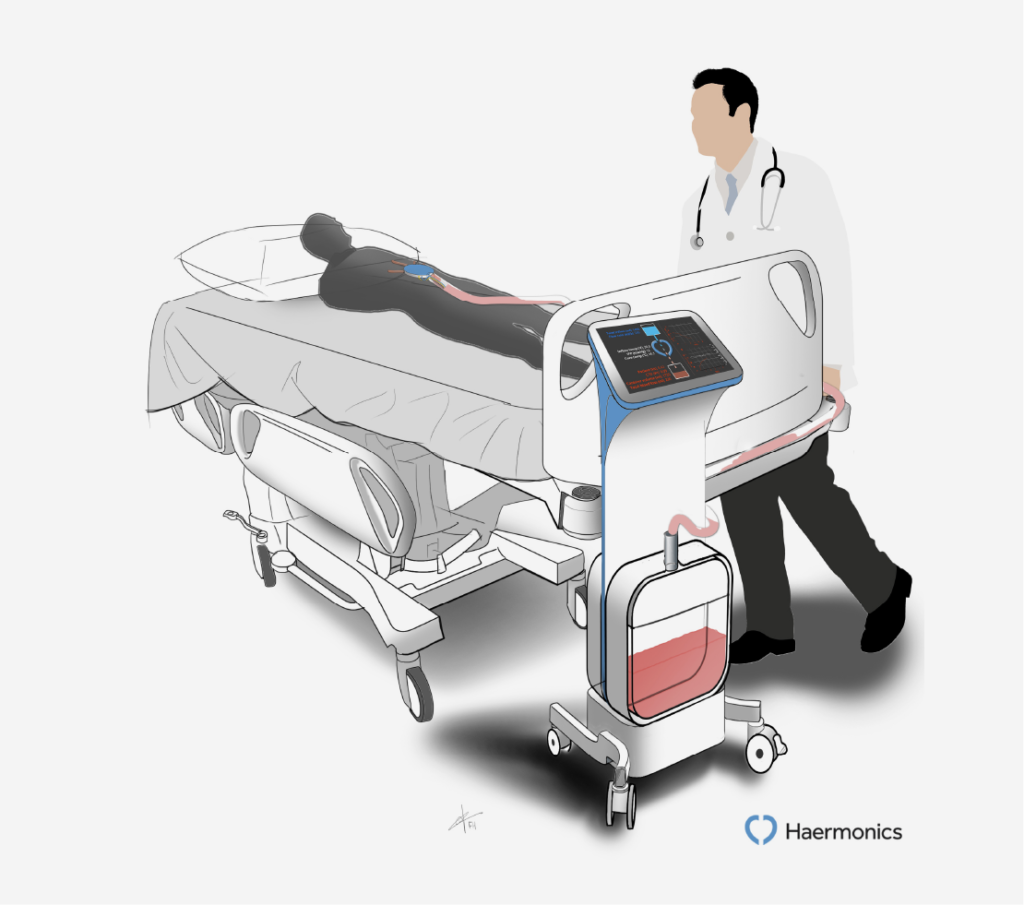
Haermonics Flush
THINC. in collaboration with Haermonics and clinical experts developed a randomized clinical trial to determine the effects of using Haermonics Flush on the reduction of blood loss and related complications.
Haermonics has developed a medical device that can flush the pericardial space with a warm saline solution to stop bleeding after open-heart surgery, called the Haermonics Flush. Haermonics technology allows controlled infusion of a heated liquid solution into the pericardial cavity after surgery, while the system monitors blood loss and pressure in the pericardial cavity. Continuous flushing will result in a lower-viscosity mixture that prevents thoracic tubes from clogging and promotes evacuation of blood and blood clots from the pericardial cavity.

METHODOLOGY CLINICAL STUDY
We offer consultancy and advisory activities in setting up a clinical study and writing a study protocol. We can help to answer questions like: What would be the most efficient and appropriate design for the study to validly answer my research question? What aspects need to be covered by the study protocol to comply with rules and regulations? How many participants do I need?

CLINICAL STUDY
In a clinical study, the (clinical) performance, efficacy, effectiveness and/or efficiency of the innovation will be studied. In designing and performing the clinical study, several elements need to be addressed such as the need for a control group, randomization, follow-up time. We will consider the regulatory and ethical requirements as well as the logistics.
FrankySpeaking
FrankySpeaking
THINC. conducted an innovation assessment in which the innovation and its components were fully vetted, the mechanism of action was determined, clinical experts were consulted, current literature was mapped to identify knowledge gaps, and suggestions were made to fill these gaps with additional research. In addition, a pilot study was designed with FrankySpeaking and experts in the field of EMDR to gain further insight into the usability, feasibility and early efficacy of FrankySpeaking whether or not in combination with EMDR.
FrankySpeaking combines a new invention with an already existing therapy, EMDR. By stabilizing the jaw, peace is brought to the oral cavity and EMDR neutralizes negative memories.

CLINICAL STUDY
In a clinical study, the (clinical) performance, efficacy, effectiveness and/or efficiency of the innovation will be studied. In designing and performing the clinical study, several elements need to be addressed such as the need for a control group, randomization, follow-up time. We will consider the regulatory and ethical requirements as well as the logistics.

METHODOLOGY CLINICAL STUDY
We offer consultancy and advisory activities in setting up a clinical study and writing a study protocol. We can help to answer questions like: What would be the most efficient and appropriate design for the study to validly answer my research question? What aspects need to be covered by the study protocol to comply with rules and regulations? How many participants do I need?
Sapien 3
Sapien 3
THINC. identified prognostic factors for early or late discharge from the hospital in TAVI patients, and developed and internally validated a prognostic model to predict early or late discharge in these patients.
Edwards has developed a Transcatheter Aortic Valve Implantation (TAVI) that can be used in patients with severe aortic stenosis. TAVI is expected to provide reduced risk of complications and associated length and cost of admission. Yet little is known about predictors of early discharge and whether it can be reliably predicted.

METHODOLOGY CLINICAL STUDY
We offer consultancy and advisory activities in setting up a clinical study and writing a study protocol. We can help to answer questions like: What would be the most efficient and appropriate design for the study to validly answer my research question? What aspects need to be covered by the study protocol to comply with rules and regulations? How many participants do I need?
WeQare

WeQare
THINC. supports SmartQare in obtaining CE marking within the Medical Device Regulation (MDR): Formative and Summative Usability Testing.
SmartQare is developing weQare, a wearable multisensor solution for 24-hour remote monitoring of at-risk individuals. In addition to monitoring vital signs, it can detect falls and offers the option of manual alarms. A tool that puts people first and helps healthcare professionals organize the care process around patients effectively and efficiently. The solution consists of: 1) a comfortable wearable medical device with various sensors. 2) an online platform (CDSS) that receives the measured values and processes them in a secure pseudonymized manner. 3) a mobile app.

USABILITY TESTING
In a usability test we evaluate the usability and user friendliness of an innovation with end users. Using for example (video) observation and qualitative research, we will be able to quantify how users actually interact with and experience the innovation. This study will give recommendations for optimization and further implementation.
Untire

Untire
THINC. is advising on and designing a clinical randomized trial to evaluate the effect of the Untire app in Germany in order to subsequently gain access for Untire (through BfArM) to the German market.
Tired of Cancer has developed an app (Untire) that helps (former) cancer patients cope with extreme fatigue. Untire was named 1 of the best 5 apps within oncology worldwide by ORCHA (renowned organization where it talks about rating digital care), out of a total of 3603 apps rated.

METHODOLOGY CLINICAL STUDY
We offer consultancy and advisory activities in setting up a clinical study and writing a study protocol. We can help to answer questions like: What would be the most efficient and appropriate design for the study to validly answer my research question? What aspects need to be covered by the study protocol to comply with rules and regulations? How many participants do I need?
Hervitas

Hervitas
THINC. has conducted a retrospective survey evaluation among former clients of Hervitas. In addition, THINC. is currently conducting a feasibility or feasibility study at Hervitas looking at the preliminary effects of the treatment. Both studies are made possible by Dutch Lottery.
Hervitas is a specialized mental health institution that offers a specific treatment program for gaming and gambling disorder. When someone suffers from problematic gambling, that person experiences physical, psychological, and social problems AND continues to play.

FEASIBILITY TESTING
A feasibility study assesses the acceptance of an innovation for a longer period of usage, in the intended context. Using a mixed method of qualitative and quantitative methods, several aspects will be measured, such as: acceptance, interest, implementation, practicability, adaptation, integration, expansion and preliminary effectiveness.

DEMONSTRATION OF THE MECHANISM OF ACTION
In this analysis a causal path will be drawn to clarify how the innovation will result in the intended positive effects. These pathways are crucial to determine consecutive studies and relevant outcomes to study to eventually demonstrate the benefits of the innovation.
BreasTel®
BreasTel®
THINC. is currently investigating for IDS International B.V. the improvement potential and market opportunities for BreasTel® in countries and settings with reduced access to mammography and is doing so by conducting literature reviews and expert interviews.
BreasTel® is a compact portable ultrasound device under development by Dutch start-up IDS International B.V, which helps characterize a palpable lump in the breast. BreasTel® is specifically designed to be used by untrained healthcare workers or women. The goal of BreasTel® is to develop a low-cost user-friendly modality that can be used for Point-of-Care testing of a palpable lump in the breast. It uses ultrasound technology combined with artificial intelligence (AI) and, without requiring personal interpretation, distinguishes between benign and suspicious tumors that may indicate breast cancer. The intention is for BreasTel® to support breast cancer diagnostics and help select where a referral for further diagnostics (such as mammography) is needed.

LITERATURE REVIEW
Using a systematic literature review, we will identify the current scientific evidence that can substantiate the mechanism of action of the innovation. This will provide the innovator an idea of the theoretical chance of the innovation to be effective and as such to further build the business case. Additionally, it provides valuable input for what studies are needed to fill the identified gaps in the knowledge base to further substantiate the mechanism of action.

APPLICATION IDENTIFICATION
What would be the best context to apply the innovation? In which patient group or care pathway will the innovation generate the most impact? During this application identification we will introduce the innovator to experts in potential application areas to understand how they would welcome this type of innovation in their care processes and to eventually identify the most favorable context of application.
Fast Focus

Fast Focus
THINC mapped what the clinical potential or mechanism of action might be, following an exploration of the application areas of FastFocus EarSensor. Through a literature review on monitoring vital signs and (group) interviews with experts (scientists, pulmonologists, nurses and a physical therapist), THINC. gathered information and identified four applications (such as, for example, continuous monitoring in the hospital, self-management or replacing manual actions).
FastFocus is a Wireless Patient Monitoring System: a device specifically designed for wireless and continuous measurement of patients' vital signs, posture and locomotor activity. The device is worn on the ear (see image).

LITERATURE REVIEW
Using a systematic literature review, we will identify the current scientific evidence that can substantiate the mechanism of action of the innovation. This will provide the innovator an idea of the theoretical chance of the innovation to be effective and as such to further build the business case. Additionally, it provides valuable input for what studies are needed to fill the identified gaps in the knowledge base to further substantiate the mechanism of action.

USABILITY TESTING
In a usability test we evaluate the usability and user friendliness of an innovation with end users. Using for example (video) observation and qualitative research, we will be able to quantify how users actually interact with and experience the innovation. This study will give recommendations for optimization and further implementation.
Mind Mansion
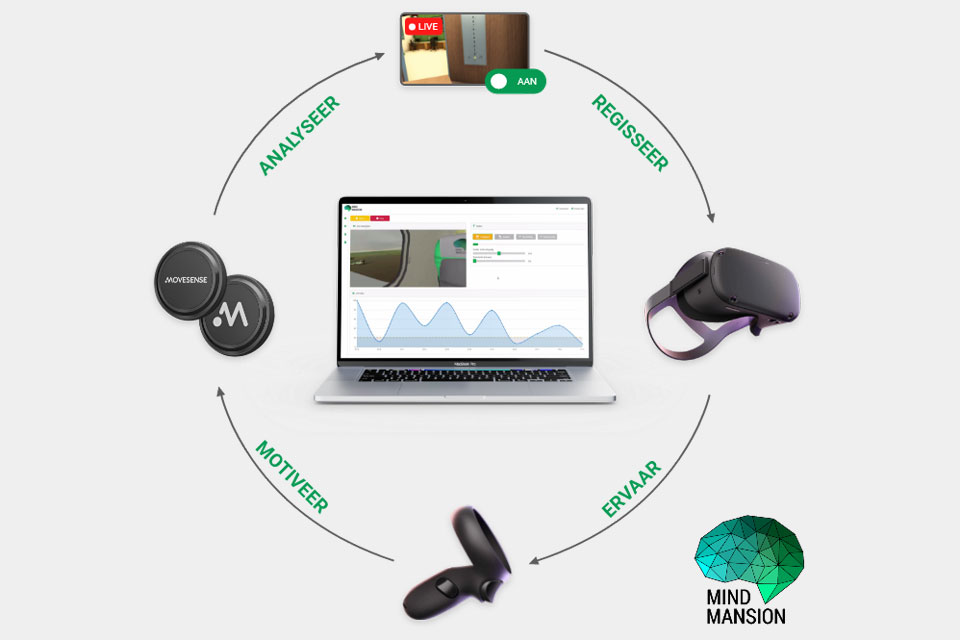
Mind Mansion
THINC. is conducting an early feasibility study for Mind Mansion to understand the usability, usability and implementation potential of their innovation.
About 1 million people in the Netherlands struggle with an anxiety disorder. This can lead to serious obstacles in daily life. An effective treatment for this is exposure therapy. The startup Mind Mansion developed an innovation to help people overcome their anxiety through virtual reality exposure therapy.

DEMONSTRATION OF THE MECHANISM OF ACTION
In this analysis a causal path will be drawn to clarify how the innovation will result in the intended positive effects. These pathways are crucial to determine consecutive studies and relevant outcomes to study to eventually demonstrate the benefits of the innovation.

FEASIBILITY TESTING
A feasibility study assesses the acceptance of an innovation for a longer period of usage, in the intended context. Using a mixed method of qualitative and quantitative methods, several aspects will be measured, such as: acceptance, interest, implementation, practicability, adaptation, integration, expansion and preliminary effectiveness.
Philips Healthcare
Philips Healthcare
In samenwerking met Philips heeft THINC. predictiemodellen gecombineerd met klinische en economische uitkomsten. Deze dienst geeft inzicht in de klinische en monetaire voordelen van innovaties. Uitkomsten van dergelijke onderzoeken worden gebruikt voor management besluiten op het gebied van toekomstige research & development activiteiten. THINC. is daarnaast partner van Philips HealthWorks. Philips HealthWorks creëert samenwerkingsmogelijkheden tussen Philips en bedrijven in een vroeg stadium, evenals ecosysteempartners, om innovatie in de gezondheidszorg te versnellen.
Philips is a health technology company. Philips focuses on improving health and enhancing health outcomes across the healthcare spectrum, from healthy living and prevention, to diagnosis, treatment and home care. Philips uses advanced technology and deep clinical and consumer insights to deliver integrated solutions.

METHODOLOGY CLINICAL STUDY
We offer consultancy and advisory activities in setting up a clinical study and writing a study protocol. We can help to answer questions like: What would be the most efficient and appropriate design for the study to validly answer my research question? What aspects need to be covered by the study protocol to comply with rules and regulations? How many participants do I need?
Oncode Institute

Oncode Institute
THINC. collaborates with Oncode on multiple research topics, where we identify at an early stage what the added value (clinical and economic) of ideas and innovations can be.
Oncode's vision is "Outsmarting cancer, impacting lives. Oncode is an independent institute focused on understanding and researching cancer and translating findings into applications for practice. In addition to conducting fundamental research, Oncode specializes in collaborations with parties to translate research findings from clinical researchers, into further research, new diagnostics, new treatments and new pharmaceutical solutions.

EARLY HTA
In an early Health Technology Assessment (HTA) we will research the minimal economic requirements for the innovation to bring added value, compared to the current standard of care. We will focus on the intended costs and the intended effects of an innovation. An early HTA provides an early estimation of the change in healthcare costs after implementing the innovation.
Docly
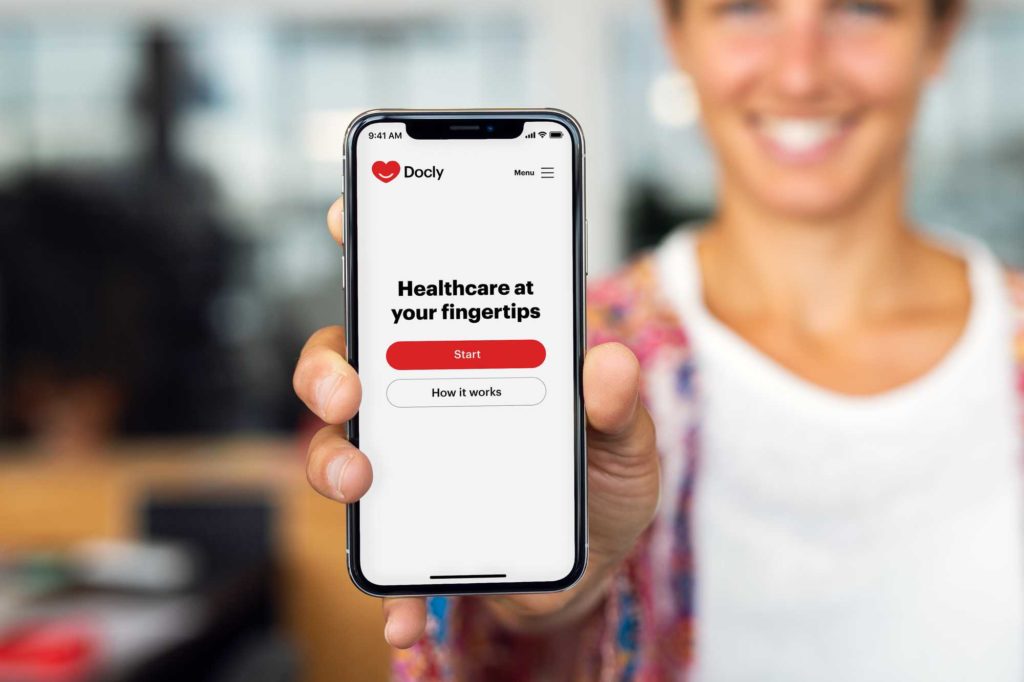
Docly
THINC. is conducting a Content Validity, Face Validity and Feasibility study of the Docly system in Huisartsenpost Eemland. THINC. finds out how Docly is received by patients and professionals, whether the system corresponds to the current GP standard, how Docly fits into the care process and how it leads to more efficient GP care in the Netherlands. We do this through interactive sessions, qualitative research, quantitative research and collaborations with general practitioners and specialists.
Docly is driving the digitization of primary care in Sweden and has already treated over 400,000 patients in Sweden. Docly offers an online service where the patient has direct access to a GP via their cell phone. The platform offers a solution for the patient's entire pathway: automated triage and history, interaction between doctor and patient via messenger (possibly combined with calling or video calling), diagnostics (including decision support) and referral.

FEASIBILITY TESTING
A feasibility study assesses the acceptance of an innovation for a longer period of usage, in the intended context. Using a mixed method of qualitative and quantitative methods, several aspects will be measured, such as: acceptance, interest, implementation, practicability, adaptation, integration, expansion and preliminary effectiveness.

CONTEXT & CURRENT PRACTICE ANALYSE
During this analysis, an independent portfolio will be built that clarifies which (unmet) needs will be addressed with the innovation. Using literature research, interviews and focus groups it will be assessed which problems can be addressed by the innovation, and how this compares to the current standard.
DeltaScan
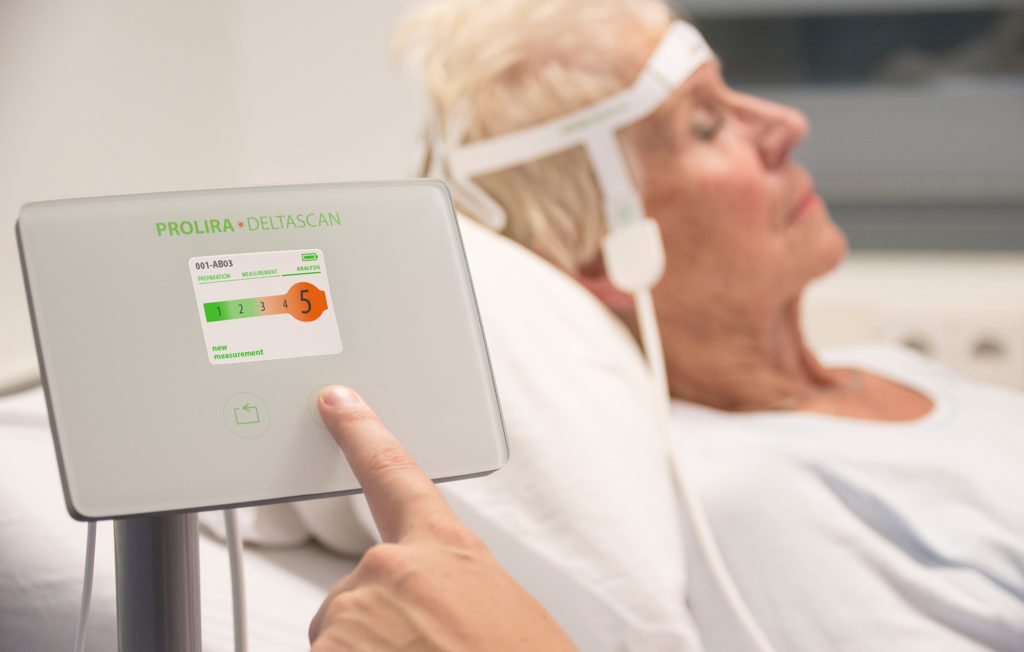
DeltaScan
THINC. in collaboration with Prolira, is conducting a cost-effectiveness study and extensive literature review, among other things, and is providing methodological support to Prolira in the design and conduct of clinical studies required for CE-/FDA approval.
The DeltaScan, developed by the company Prolira, is the world's first objective measuring instrument that can detect delirium (sudden onset of confusion). The DeltaScan enables early detection and aims to reduce the (severe) consequences of delirium.

METHODOLOGY CLINICAL STUDY
We offer consultancy and advisory activities in setting up a clinical study and writing a study protocol. We can help to answer questions like: What would be the most efficient and appropriate design for the study to validly answer my research question? What aspects need to be covered by the study protocol to comply with rules and regulations? How many participants do I need?

HTA
In this extensive quantitative analysis, we will use prospective study data, literature data and expert opinion. Optionally we develop a model to answer several cost effectiveness questions or scenarios. For example: what are the additional costs per gained life year? Does the prevention of a complication result in cost savings?

LITERATURE REVIEW
Using a systematic literature review, we will identify the current scientific evidence that can substantiate the mechanism of action of the innovation. This will provide the innovator an idea of the theoretical chance of the innovation to be effective and as such to further build the business case. Additionally, it provides valuable input for what studies are needed to fill the identified gaps in the knowledge base to further substantiate the mechanism of action.
FREE Bionics

FREE Bionics
THINC. collaborates with de Hoogstraat and FREE Bionics in establishing the research protocol and going through the appropriate research guidelines according to Dutch standards.
FREE Bionics is a Taiwanese company made up of researchers, engineers and technicians. FREE Bionics has developed the FREE Walk, an exoskeleton for patients with spinal cord injury, nerve damage or weakened limbs. The FREE Walk is currently being investigated during a clinical trial at Rehabilitation Center de Hoogstraat.

METHODOLOGY CLINICAL STUDY
We offer consultancy and advisory activities in setting up a clinical study and writing a study protocol. We can help to answer questions like: What would be the most efficient and appropriate design for the study to validly answer my research question? What aspects need to be covered by the study protocol to comply with rules and regulations? How many participants do I need?
MRIguidance
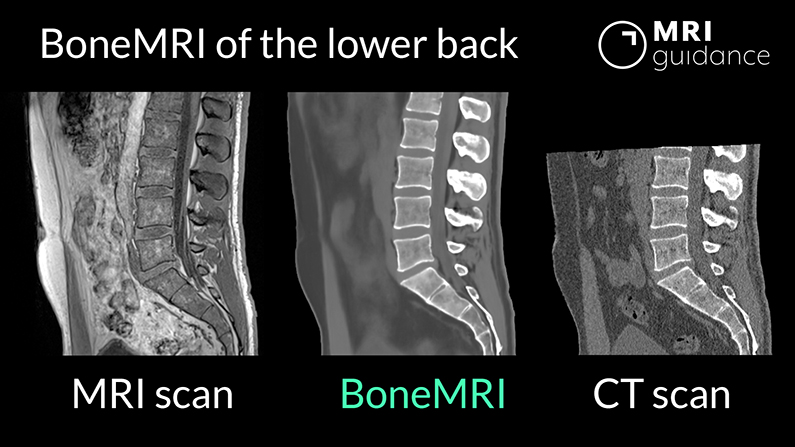
MRIguidance
THINC. established the methodology of the clinical trial in collaboration with MRIguidance.
MRIguidance is a UMC Utrecht spin-off company that developed the medical imaging technology BoneMRI. BoneMRI is software that can extract CT image-related information from an MRI scan, adding 3D visualization of bones to an MRI scan. This innovation aims to provide better diagnostic information, prevent radiations and thus develop a more efficient care path for patients and professionals.

METHODOLOGY CLINICAL STUDY
We offer consultancy and advisory activities in setting up a clinical study and writing a study protocol. We can help to answer questions like: What would be the most efficient and appropriate design for the study to validly answer my research question? What aspects need to be covered by the study protocol to comply with rules and regulations? How many participants do I need?
Release

Release
THINC. developed a model for maximum utilization of routine care data that creates a continuous learning environment in order to identify more and more clearly which intervention components (WHAT) work for which profile of patients (WHO) and in which type of care model/context (HOW and WHERE). This constant flow of knowledge parallel to the natural rollout of eHealth provides essential input for quality improvement and is also a catalyst for new innovations.
Several challenges stand in the way of realizing the true potential of e-health in Heart Failure patients: insufficient evidence base, heterogeneity of technologies and lack of guidance and quality control/improvement. The goal of the RELEASE-HF (REsponsible roLl-out of E-heAlth through Systematic Evaluation - Heart Failure) is to achieve a responsible and safe roll-out and continuous quality improvement of e-health/tele guidance in HF.

METHODOLOGY CLINICAL STUDY
We offer consultancy and advisory activities in setting up a clinical study and writing a study protocol. We can help to answer questions like: What would be the most efficient and appropriate design for the study to validly answer my research question? What aspects need to be covered by the study protocol to comply with rules and regulations? How many participants do I need?
Safe@home

Safe@home
Pregnant women who have had a complication in a previous pregnancy such as preeclampsia, high blood pressure or women who are pregnant and have heart problems need extra monitoring. In the current healthcare system, this high-risk group is asked to undergo regular outpatient examinations. These checkups are stressful for the women and involve high costs. Safe@home is a home monitoring system. With Safe@home, women can use monitoring equipment at home and be monitored remotely (by the doctor). This offers pregnant women a more comfortable care path, and doctors the chance to spot any abnormalities early.
For this innovation, we conducted a baseline measurement in care consumption (outpatient visits, ultrasounds, etc.). This measurement answers the question: how much care is currently needed to properly monitor this high-risk group? This baseline measurement will allow researchers to determine the difference in care utilization when Safe@home is implemented.

EARLY HTA
In an early Health Technology Assessment (HTA) we will research the minimal economic requirements for the innovation to bring added value, compared to the current standard of care. We will focus on the intended costs and the intended effects of an innovation. An early HTA provides an early estimation of the change in healthcare costs after implementing the innovation.
GGZ Central

GGZ Central
THINC. is working with GGZ Centraal on a Health Technology Assessment that analyzes what will change in total healthcare costs if this group of patients starts using this activity program, in both the short and long term. In this analysis, we use data collected by GGZ Centraal in a study and data obtained through literature review (important data in this study include costs of the activity program, residential form, medication use, dietician support).
Research has shown that patients admitted to mental health institutions for long periods of time are more likely to suffer from obesity and related conditions such as diabetes and cardiovascular disease. GGZ Centraal has developed an activity program to combat the development of these conditions.

HTA
In this extensive quantitative analysis, we will use prospective study data, literature data and expert opinion. Optionally we develop a model to answer several cost effectiveness questions or scenarios. For example: what are the additional costs per gained life year? Does the prevention of a complication result in cost savings?
NightWatch

NightWatch
THINC. is collaborating with UMC UTRECHT, SEIN and Kempenhaeghe in the PROMISE study. In this extensive project we measure how the NightWatch can be used to support children with epilepsy and their parent(s)/caregivers at home. THINC. is conducting the feasibility study.
The NightWatch is a detection system for people with severe epilepsy. The NightWatch can be worn on the arm and signals when an epileptic seizure occurs.

FEASIBILITY TESTING
A feasibility study assesses the acceptance of an innovation for a longer period of usage, in the intended context. Using a mixed method of qualitative and quantitative methods, several aspects will be measured, such as: acceptance, interest, implementation, practicability, adaptation, integration, expansion and preliminary effectiveness.
Proton Therapy
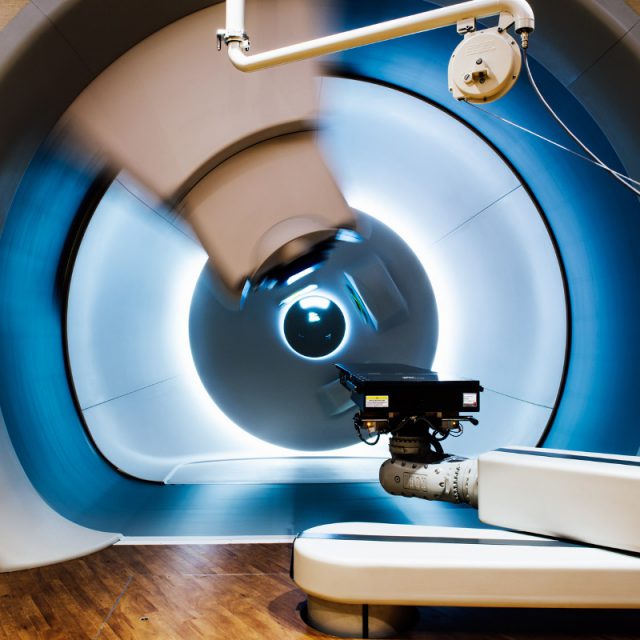
Proton Therapy
THINC, together with radiotherapists from proton and non-proton centers, validates and optimizes radiation damage prediction models in order to select patients who will benefit most from proton therapy. A unique approach regarding the introduction of proton therapy in the world making optimal use of existing knowledge and a fair and transparent rationale for selection.
Since 2018, the first patients have been treated with proton therapy in the Netherlands. This is a new way of radiating cancer that leads to less collateral damage than the current standard, photon therapy.

METHODOLOGY CLINICAL STUDY
We offer consultancy and advisory activities in setting up a clinical study and writing a study protocol. We can help to answer questions like: What would be the most efficient and appropriate design for the study to validly answer my research question? What aspects need to be covered by the study protocol to comply with rules and regulations? How many participants do I need?
Wind Tales

Wind Tales
Wind Tales is a breath-controlled game (serious game) designed for children with Cystic Fibrosis. The digital game enables children to use breathing exercises to control an avatar to steer and overcome obstacles in a fantasy world. In close collaboration with Wind Tales, we performed a usability and a feasibility study for their serious game. The goal of these studies was to enable Wind Tales address the opportunities and threats as perceived by the end users with the goal to achieve a flawless implementation of the game.

FEASIBILITY TESTING
A feasibility study assesses the acceptance of an innovation for a longer period of usage, in the intended context. Using a mixed method of qualitative and quantitative methods, several aspects will be measured, such as: acceptance, interest, implementation, practicability, adaptation, integration, expansion and preliminary effectiveness.
Chris van Lieshout
For modeling care pathways and finding and analyzing data, go to Chris.
After studying Health Sciences at the University of Twente, Chris has been working at THINC. in the Health Technology Assessment team since the summer of 2017. Chris has worked on a wide variety of projects, investigating the added value of the innovation for innovators. With his pragmatic nature, he is able to quickly and efficiently arrive at new health and economic insights.
And further? Regularly a run, weekends out into the wide world and drinks or dinners with friends.
Elke Mathijssen
An investigation that must be carried out quickly but carefully? Then you have come to the right place at Elke.
Within THINC. Elke is mainly engaged in research into the usability and feasibility of healthcare innovations. Because of her background in nursing, she knows the ins and outs of clinical practice and how to place herself in the shoes of the end users of a healthcare innovation. With her eye for detail, she strives to ensure that everything is executed to perfection within the desired time frame.
And further? In 2020, Elke received her doctorate as a scientist and was also promoted to mother. She enjoys the center of Arnhem and the nature around it.
Gerdi Janssen
Connecting and looking for opportunities. That is what Gerdi is committed to. Relationships who wants to take their innovation process a step further, matching their needs to the expertise of THINC. resulting in a good cooperation and final result.
Starting in strategic research, Gerdi is experienced in consultative selling where she connected companies with expertise in strategy, business development or corporate social responsibility. She is experienced with clients in the field of Food, Health, International business and Inclusion and Diversity.
In private she does interior and gardening designing and loves to create is too. She likes running as sport and chatting and having a drink with family and friends. She also likes travelling to different countries to enjoy new cultures and nature.
Hans Reitsma
Looking for tailored advice for your clinical study?
After his medical exam and academic promotion, Hans consciously opted for clinical epidemiology. Demonstrating the added value of all kinds of new innovations is varied and challenging. Hans is well informed of all new regulations and methodological developments. In addition, he actively and creatively thinks along with innovators about the best step forward for their innovation.
And further? Fly fishing! Hans likes to stroll along the waterfront and can also be found with his family for whom he likes to cook extensively.
Ingelin Kvamme
Along with a background in economics and specialization in Health Economics at Erasmus University Rotterdam, Ingelin has worked with Health Technology Assessment since 2020. As such, she gained experience in conceptualization, building, and adaption of Health Economic modelling. Combining her passion for client satisfaction and academic background, Ingelin works to ensure quality and efficiency in cost-effectiveness analyses for innovators.
Of professional interest, Ingelin is particularly interested in HTA capacity building, while on a personal note, Ingelin enjoys trail running, social and cultural studies, reading, and farming.
Jaap Trappenburg
Making the complex simple – Jaap quickly finds out the cause of your innovation challenge(s) and translates it into concrete tailor-made solutions.
Healthcare innovation research is in his genes. In his position as Associate Professor, he coordinates a line of research into innovative solutions within chronic care. Within THINC. he leads a team of researchers who mainly focus on the early evaluation of innovations in the real world. Think of user research (usability studies) and early feasibility or effectiveness studies (feasibility / pilot studies).
And further? "Never a dull moment". Farm La Trappe houses a family, animals and DIY projects. Furthermore, cycling, skating, festivals and friendships are favorite.
Katharina Abraham
For capturing your innovation’s health & economic value across stakeholders, go to Katharina.
After studying Health Economics at Erasmus University Rotterdam, Katharina has been gathering experience in the conceptualization, building and adaptation of health economic models since 2018.
After studying Health Economics at Erasmus University Rotterdam, Katharina has been gathering experience in the conceptualization, building and adaptation of health economic models since 2018. A special focus of hers lies on the inclusion of the wider economic, clinical and health aspects impacted by the innovation. With her academic background and pragmatic nature, she knows how to balance the quality and efficiency aspect of conducting cost-effectiveness analyses for innovators.
Besides her analytical work, Katharina is spending her time training JiuJitsu, lifting weights, reading books, and learning Java programming.
Kim Romijnders
Kim werkt als onderzoeker gezondheidsgedrag bij het Julius Centrum voor Gezondheidswetenschappen en Eerstelijnszorg. Met behulp van kwalitatief en survey onderzoek richt Kim zich op het patiëntenperspectief, gezondheidsgedrag, seksuele gezondheid en gezondheidsbeslissingen in relatie tot infectieziekten.
Ze wil met haar onderzoek een impact maken die ertoe doet door de mening, het perspectief en de ervaring van patiënten in de voorhoede van de gezondheidszorg en het wetenschappelijk onderzoek te plaatsen.
Momenteel werkt ze aan multidisciplinaire projecten samen met patiëntenorganisaties, zoals de vereniging Ditch HIV, om de perceptie van mensen met hiv te betrekken bij onderzoek naar hiv-behandeling en preventie.
Mieke Arnoldus
Innovators helpen om inzicht te krijgen in de toegevoegde waarde van hun zorginnovaties met behulp van kosteneffectiviteitsanalyses doet Mieke graag.
Na het afronden van haar bachelor Gezondheidswetenschappen gefocust op innovatie aan de Universiteit Twente, heeft ze tijdens haar Europese master Health Economics & Management diverse Europese invalshoeken op het gebied van gezondheid verkend. De kennis en ervaringen die ze heeft opgedaan in diverse zorgsystemen past ze nu toe in de Nederlandse context.
Daarnaast staat ze graag samen met haar team op het basketbalveld en experimenteert ze graag met nieuwe recepten voor familie en vrienden.
Peter Gisberts
“Succes bouw je samen, en er is niks mooiers dan aan een succesvol team te bouwen”
Peter is een ervaren business Healthcare leider, met internationale ervaring. Met THINC Healthcare denkt Peter graag mee met Health innovators hoe hun innovatieve oplossingen de grootste impact kunnen maken in het verbeteren van het leven patiënten.
Hij heeft jaren lang gewerkt voor verschillende grote farma en diagnosticekbedrijven in de rol van Medical Affairs, en Market Access Director. In 2021 is hij gestart in het UMC Utrecht als Directeur van U-TRIAL, the Utrecht TRial Alliance, waar hij samen met het U-TRIAL team zich inzet voor het versterken van het klinische onderzoeksklimaat binnen het UMC Utrecht.
En verder? Peter loopt regelmatig een halve of hele marathon, reist graag, vooral in Azie, en Zuidelijk Afrika, en is daarnaast een bevlogen coach van het hockey team van zoon.
Stijn Boeren
Good with numbers and a big interest in everything that has to do with research and medicine
After gaining several years of experience at UMC Utrecht, Stijn can now combine the clinical knowledge he gains during his studies in Medicine with his master's degree in Health Economics. Because of this combination, he knows how to make the right link between clinical practice and the models.
And besides HTA? As a great music lover, Stijn likes to play the piano or guitar and visits many concerts and festivals with his friends.
Taylie Hu
Hands-on mentality. Taylie signals and ensures appointments are kept.
After completing her master's degree in neuropsychology, she began to study holistic medicine and that is how Ayurveda came her way. Her innate entrepreneurial spirit, has allowed her to gain experience in various fields. Besides setting up her own coaching practice, she has years of experience in business services.... Driven, open minded and curious for new challenges, she has taken on the role as Office Manager at THINC
Service-oriented work combined with scientific research makes Taylie feel right at home.
What else? Taylie likes to drink a glass of red in the winter and a glass of white in the summer.
Hiking through nature, boxing classes and innovative ideas are things that make her happy.
Tijn Stolk
Tijn has a solid scientific background after completing the Bachelor of Biomedical Sciences and the Master of Cognitive Neuroscience. During his study career, he experienced the pitfalls of the Dutch healthcare system with internships and jobs in several hospitals. With his well-developed analytical and communicative skills, Tijn is highly motivated to be engaged in innovating the healthcare system. The combination of his motivation, background and interest in ‘healthtech’ guided him to THINC., where he will mainly be focusing on the evaluation on clinical efficacy and feasibility of innovations.
Furthermore, Tijn is passionate about sports. He especially likes long-distance running and road cycling. Besides, he likes seeking adventure in the mountains or by scuba diving on special places.
Wendela de Lange
Designing and executing research, in practice, with healthcare innovations that really benefit people, that's what Wendela gets out of bed for every day!
As a nurse, Wendela worked in the hospital for 10 years, where the challenge for her was always to provide the best care, at the right time and tailored to each individual. After graduating in Clinical Health Sciences, she made the move to research. Her clinical experience is key for her research practice. Her focus is on understanding how care works, how it can connect to and have an impact on people's lives.
And further? Wendela is a real people person and can be found with her family and friends or doing voluntary work. Meanwhile, she captures the ultimate memory with her camera.

Beste THINC. relatie,
Langs deze weg willen we u meedelen dat het UMC Utrecht heeft besloten te stoppen met de activiteiten van THINC. (The Healthcare Innovation Center). Dit betekent dat er na zeven jaar een einde komt aan ons werk als een science as a service organisatie.
Impact op innovatie
In de afgelopen jaren hebben we met trots meer dan 150 bedrijven – variërend van start-ups tot multinationals – een flinke stap verder geholpen in hun uitdagende innovatieprocessen.Zo hebben we talloze innovaties geholpen met effectiever en doelmatiger worden, toetreding tot de markt, of succesvolle implementatie.
Wetenschappelijk pionieren
THINC. heeft zich onderscheiden door wetenschappelijk, kort cyclisch en multidisciplinair innovatieonderzoek. We hebben nieuwe paden bewandeld en standaarden neergezet in formats, organisatie en methodologie. We zijn er trots op dat we hebben bijgedragen aan het definiëren van de normen voor toekomstig innovationderzoek in de sector.Dankbaar
Onze waardering gaat uit naar de zorginnovators die hun vertrouwen in ons hebben gesteld. Grote dank gaat ook uit naar alle medewerkers van THINC. Hun collegialiteit, professionaliteit en onvermoeibare inzet hebben altijd het hart van THINC. gevormd.Bedankt voor jullie vertrouwen, samenwerking en toewijding.
Met vriendelijke groet,
Peter Gisberts
Managing Directeur THINC.
Voor verdere vragen kunt u contact opnemen met Peter Gisberts (p.g.l.gisberts@umcutrecht.nl)
----- English ------------------
Dear THINC. Relationship,
We would like to inform you that the UMC Utrecht has decided to stop the activities of THINC. (The Healthcare Innovation Center). This means that after seven years our work as a science as a service organization will come to an end.
Impact on innovation
In recent years, we are proud to have helped more than 150 companies – ranging from start-ups to multinationals – to take a big step forward in their challenging innovation processes. We have helped countless innovations become more effective and efficient, enter the market, or be successfully implemented.Scientific pioneering
THINC. has distinguished itself through scientific, short-cycle and multidisciplinary innovation research. We have blazed new trails and set standards in formats, organization and methodology. We are proud to have helped define the standards for future innovation research in the sector.Gratitude
Our appreciation goes out to the healthcare innovators who have placed their trust in us. A big thank you also goes to all THINC employees. Their collegiality, professionalism and tireless efforts have always formed the heart of THINC.Thank you for your trust, cooperation and dedication.
Yours sincerely,
Peter Gisberts
Managing Director THINC.
For further questions, please contact Peter Gisberts (p.g.l.gisberts@umcutrecht.nl)

Beste THINC. relatie,
Langs deze weg willen we u meedelen dat het UMC Utrecht heeft besloten te stoppen met de activiteiten van THINC. (The Healthcare Innovation Center). Dit betekent dat er na zeven jaar een einde komt aan ons werk als een science as a service organisatie.
Impact op innovatie
In de afgelopen jaren hebben we met trots meer dan 150 bedrijven – variërend van start-ups tot multinationals – een flinke stap verder geholpen in hun uitdagende innovatieprocessen.Zo hebben we talloze innovaties geholpen met effectiever en doelmatiger worden, toetreding tot de markt, of succesvolle implementatie.
Wetenschappelijk pionieren
THINC. heeft zich onderscheiden door wetenschappelijk, kort cyclisch en multidisciplinair innovatieonderzoek. We hebben nieuwe paden bewandeld en standaarden neergezet in formats, organisatie en methodologie. We zijn er trots op dat we hebben bijgedragen aan het definiëren van de normen voor toekomstig innovationderzoek in de sector.Dankbaar
Onze waardering gaat uit naar de zorginnovators die hun vertrouwen in ons hebben gesteld. Grote dank gaat ook uit naar alle medewerkers van THINC. Hun collegialiteit, professionaliteit en onvermoeibare inzet hebben altijd het hart van THINC. gevormd.Bedankt voor jullie vertrouwen, samenwerking en toewijding.
Met vriendelijke groet,
Peter Gisberts
Managing Directeur THINC.
Voor verdere vragen kunt u contact opnemen met Peter Gisberts (p.g.l.gisberts@umcutrecht.nl)
----- English ------------------
Dear THINC. Relationship,
We would like to inform you that the UMC Utrecht has decided to stop the activities of THINC. (The Healthcare Innovation Center). This means that after seven years our work as a science as a service organization will come to an end.
Impact on innovation
In recent years, we are proud to have helped more than 150 companies – ranging from start-ups to multinationals – to take a big step forward in their challenging innovation processes. We have helped countless innovations become more effective and efficient, enter the market, or be successfully implemented.Scientific pioneering
THINC. has distinguished itself through scientific, short-cycle and multidisciplinary innovation research. We have blazed new trails and set standards in formats, organization and methodology. We are proud to have helped define the standards for future innovation research in the sector.Gratitude
Our appreciation goes out to the healthcare innovators who have placed their trust in us. A big thank you also goes to all THINC employees. Their collegiality, professionalism and tireless efforts have always formed the heart of THINC.Thank you for your trust, cooperation and dedication.
Yours sincerely,
Peter Gisberts
Managing Director THINC.
For further questions, please contact Peter Gisberts (p.g.l.gisberts@umcutrecht.nl)

